Phases of Clinical Trials: What You Need to Know
It’s now more important than ever to keep informed on the processes and phases that lead to the safe deployment of drugs and vaccines. Photo: © LuckyStep/Adobe Stock.
The Covid-19 pandemic events demonstrated the importance of medical testing and pharmaceutical advancement. However, there are still a lot of misinformation flying around, especially about the details of vaccine development and clinical drug trials.
In America, the lack of information is particularly perplexing when research indicates that Americans make up 31% of clinical trial participants worldwide.
Drug tests and vaccine development help determine important details about the chemicals being manufactured. They can tell manufacturers everything from how long ibuprofen lasts to what are the potential side effects of heart medications.
But what are the processes and phases that lead to the safe deployment of drugs and vaccines? What do these phases entail and why are they important?
Let’s look at the answers to these questions.
Pre-Clinical Trial Phases
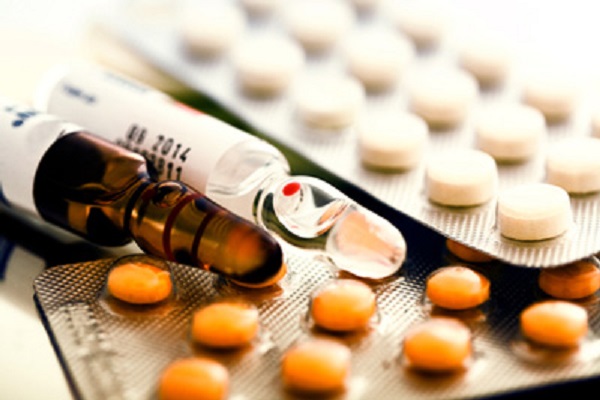
The clinical trials you probably heard a lot about are those that involve testing drugs on humans. Such testing represents only a third of the drug development process.
Before a vaccine or medication can even get that far along in development, the manufacturers would have conducted pre-clinical trial testing inside their laboratories.
There are two main categories of laboratory testing: in vivo testing and in vitro testing.
I. In Vivo Testing
In vivo testing refers to testing drugs and medication on live subjects, which are always animals. Labs are very careful about what kind of animals they use to simulate the body chemistry and physiology of humans.
For example, cancer researchers often use mice for orthotopic cancer models or inject Rhesus monkeys with vaccines to stimulate immune responses.
These tests help winnow out any major flaws and side effects before the medications even come near a human being.
II. In Vitro Testing
In vitro means "in glass" and these are the most basic tests carried out by pharmaceutical laboratories. Tissue samples and chemicals in test tubes and petri dishes are used to determine the reaction of chemicals and their effects. These glass containers are the ones that give the process its name.
In vitro tests are often used prior to in vivo testing to ensure even the animals in the next step of the tests aren't exposed to undue harm. However, they can also be used alongside in vivo testing to finetune the medications.
The 3 Phases of Clinical Trials
Phase I
After a drug clears its laboratory tests and cleared by the Food and Drug Administration, the researchers can proceed to the first step of human clinical trials. Phase I trials are very small, sometimes fewer than a 100 people are involved. Phase I trials are usually when companies offer substantial financial compensation to the participants.
A phase I trial is all about fine-tuning the drug being tested. First, it aims to determine what are the correct dosages for the drug. Researchers at this phase are looking into how your body interacts with the drug, like how long it takes to metabolize the drug and how fast your body can flush it out.
People in phase I trials are also monitored for every possible side effect and as such are put through a battery of tests.
Phase II
If a drug makes it through phase I trials, they can proceed into phase II. This phase needs a considerably larger set of participants, often hundreds or even thousands. The larger number is necessary to truly weed out the possible side effects of a particular drug.
During phase II trials, researchers seek to determine if the drug has a significant enough effect on the condition it's supposed to treat to justify phase III trials. The researchers will also be on the lookout for other side effects that may not have manifested during the first phase.
If there are side effects, this phase is also when researchers will look into methods of managing them effectively. Finally, they will use this phase to determine correct dosages more accurately.
Determining who enters phase II trials is randomized. This allows researchers to see how the drug interacts with people from all sorts of backgrounds.
Phase III
The final phase of drug development involves thousands to hundreds of thousands of participants. This is necessary to determine what side effects the drug could have if administered to the public at large. The aim of phase III trials is to see how the drug can affect the quality of life of people who take the drug.
The enormous number of participants allows researchers to see the effects of the drug in real life. They may also compare the effects of the drug compared to prevalent treatments. Most of the time, phase II trials are randomized but some medications may require specific participants.
In Conclusion
People are rightfully vigilant of clinical trials, but sometimes this vigilance is tempered by misinformation. Take it upon yourself to keep informed of the drug development process.
By learning more about these phases, you can build confidence in medicine and encourage other people to do the same.












![9 Tips for Managing Your Online Writing Projects Efficiently [node:titile]](/sites/default/files/styles/video_thumbnail_bottom/public/open-book-laptop-online-writing-tips.jpeg?itok=rI4zR3a-)






